Diabetic ketoacidosis (DKA) is one of the more dramatic problems we deal with regularly on our acute medical unit. Ryan didn’t even know he had diabetes before he came in, although the possibility had gone through his mind. He is nineteen and is studying economics at university. Last weekend he came home from college. His mother immediately noticed that he had lost weight. She thought it was because he was not eating properly – but Ryan was insistent that he ate loads. He freely admitted his diet was mainly “rubbish” – lots of burgers and chips, and he drank loads of fizzy drinks (he does not like water).

Then on Saturday night he began to get abdominal pain and vomiting. Mum thought at first that the problem was that he had eaten some undercooked sausages and had too much to drink at a party at his friend’s house the day before.
On Sunday she and Ryan’s father became really worried. He was clearly more unwell, and his breath had a really odd smell – sweet and fruity. And he was staggering to the bathroom every couple of hours to pass urine or vomit again, or both. It was when he was too ill to get to the bathroom and peed in his bed that dad called the emergency out-of-hours doctor. The GP realised straight away what was going on. Ryan was breathing deeply and heavily, dried saliva and vomit was crusted round his mouth and he was very dehydrated and sleepy. She tested his blood for glucose – the reading was “hi” – off the scale. He was soon with us in hospital.
We have a protocol for dealing with DKA which involves lots of intravenous fluids – starting with normal saline, a continuous intravenous insulin infusion, and frequent and regular checks of blood acidity, glucose and potassium. We also look for any infection which might have precipitated the DKA.
There are lots of interesting and difficult questions to ask about type 1 diabetes, such as
– what causes the pancreatic islet cells which make insulin to be destroyed?
– why does this type of diabetes run in families?
But today I am going to concentrate on two easier questions:
– why do type 1 diabetics lose weight before their disease is treated with insulin?
– what causes ketoacidosis?
When I ask “why do diabetics lose weight?” the answer I often get is that they use up fat because their bodies cannot use glucose.
This answer does not really satisfy. When we eat food our digestive system is very good at extracting energy. We are all familiar with the calorie content of food. One calorie (really kilocalorie or kcal) is the energy required to heat a litre of water by one degree centigrade. The energy content of food, measured in kilocalories or kcal, can be accurately measured by putting the burger and chips in a device known as a bomb calorimeter. This combusts the food with oxygen and measures the rise in temperature of the surrounding water bath. Let’s say the burger and chips, the three litres of cola, the cornflakes, milk and pork pie and other sundries that make up Ryan’s average daily food intake contains 2500kcal, if we were to put it in a bomb calorimeter.

Typically we absorb more than 95% of the available calories from an average meal. The only energy we cannot absorb is that in cellulose fibre*. Ryan does not eat much of that. Pretty much all of the 2500kcal he eats every day will be available as energy. Ryan is a fairly active young man. He uses 1500kcal a day just sitting studying or watching “man v food” on TV. Walking, running, cycling and energetic evenings with his new girlfriend consume the other 1000kcal. So why is he now losing weight? Weight loss means loss of fat. This is stored all around our bodies as adipose tissue. Adipose tissue contains cells that essentially only contain a large blob of triglyceride (we’ll talk about that later). Triglyceride contains nine kcal of energy per gramme, so if fat disappears, the energy must be going somewhere. The second law of thermodynamics says that energy cannot be created or destroyed.
The best way of thinking about it is that energy is going in – 2500kcal/day – and energy is going out – basal metabolic rate and energy used for exercise – also 2500kcal/day. If these are balanced, then fat stores will not change. Clearly, if Ryan is losing weight and using up fat stores there must be an imbalance with this input and output equation. The problem is not with the input, he is eating loads and absorbing the energy. It’s not with his basal metabolic rate, that is normal. It’s not with energy expenditure with exercise – that has not changed. The energy loss is in Ryan’s urine – it is full of glucose. Glucose has four kcal per gramme. He is peeing out about 800kcal per day of energy – that is why he has lost weight. It is also why he is passing so much urine and become dehydrated. Glycosuria causes an osmotic diuresis – causing him to lose extra water and be very thirsty and drink lots of fluid. Unfortunately although the fizzy drinks have lots of sugar, it is going in one end and out the other. Once the vomiting started, he was not able to keep up with the input and became even more dehydrated.
Now the ketoacidosis. This has got very little to do with glucose – it has everything to do with fat metabolism. Insulin is the hormone which controls energy metabolism. When we eat, carbohydrate is turned into glucose. Blood glucose is monitored by pancreatic islet cells. pic If glucose is high, the islet cells release insulin. Insulin has lots of important effects on how energy is used and stored.
When we are starved, with no carbohydrate intake, insulin levels drop and the lack of insulin:
1) promotes the conversion of glycogen (a glucose polymer stored in the liver) to glucose
2) keeps blood glucose levels acceptable by promoting the conversion of protein to glucose and
3) promotes breakdown of fat to provide energy.
The first two actions are important in keeping blood glucose high enough to maintain brain function– some brain cells absolutely rely on there being some glucose.
The third effect of low insulin –lipolysis – is also helpful in non-diabetics, breaking down fat to supply energy. Most tissues apart from the brain are very happy to get their energy from fat metabolism.
The problem comes when insulin is not just low, but completely absent. This never happens in non-diabetics. There is always enough insulin to keep fat breakdown under control. When insulin levels do go down to zero, in type 1 diabetics, fat starts to break down very quickly.
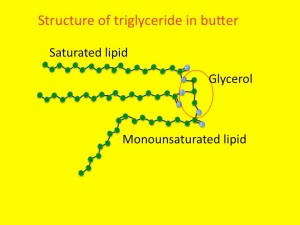
This process starts as lipolysis – the removal of the long fatty acid side chains from triglyceride. Triglyceride is an ester of fatty acid and glycerol. The glycerol backbone can be used to make more glucose (not that it is at all needed). Then the long-chain fatty acids are broken down, two carbons at a time. This is known as beta-oxidation.
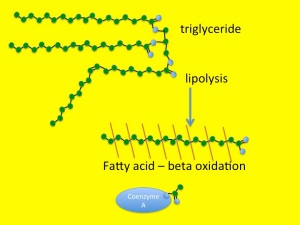
You will remember from previous posts (chest pain and horsemeat lasagne) that the carbon atom at the other end from the carboxylic acid group in a fatty acid is known as the omega carbon – hence omega 3,6 and 9 fatty acids which have double bonds in those positions. The carbon at the acid end of the molecule is the alpha carbon, and the next one along is, of course, a beta carbon. Hence beta-oxidation oxidises this carbon and removes an acetyl group from the fatty acid and attaches it to a Coenzyme A molecule. This can then be fed into the Kreb’s cycle and electron transport chain to convert it into carbon dioxide, water and nine kcal/gramme of energy in the form of ATP.
When insulin levels are very low, as was the case with Ryan, the rate of lipolysis and beta-oxidation are so fast that the Kreb’s cycle cannot keep up.

Fuel is being delivered at a faster rate than it can be burned. The irony of the situation is that there is plenty of fuel around in the form of glucose, but because it is not triggering an increase of insulin, the fat cells do not know this and keep on breaking down triglyceride. The only thing to do with all this broken down lipid is to make ketoacids (also known as ketones). In small amounts these are a quite useful alternative to glucose for energy production when we are starving – there are measurable amounts in blood and urine in healthy people if we do not eat for 12 hours or more, but low-level insulin secretion keeps the amount in check.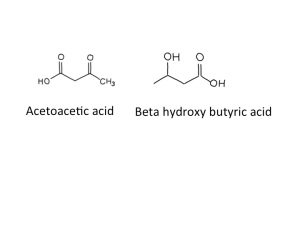
When ketoacids are made in huge amounts they cause problems because they are acidic. The two ketoacids we make are acetoacetic acid and beta-hydroxybutyric acid. Acetoacetic acid slowly, spontaneously degrades to make acetone and carbon dioxide,

which was why Ryan’s breath smelt sweet and fruity – like nail polish remover. I think that beta-hydroxy butyrate is not technically a ketone – but it is an acid. I don’t think it is necessary to make a fuss about this (but the pedant in me forced me to mention this). Acidosis makes people ill – our bodies are designed to work with a blood pH of between 7.35 and 7.45. Ryans blood pH was 7.04 when we first tested it. Many of the enzymes in our body just don’t work properly when the blood is too acid – when it becomes too severe acidosis can cause drowsiness and then coma and then death. The other problem is that the physical stress caused by ketoacidosis and dehydration from high glucose result in the release of glucocorticoids (cortisol) and adrenaline from our adrenal glands, both of which further encourage lipolysis and the formation of more ketoacids. A potentially lethal viscious cycle.
How does neutral fat make acidic substances? Well, acidity is not like water, sodium or energy where what goes in must come out. We can drink lots of acidic vinegar and it will not cause any change in blood acidity. The easiest way to look at it is that when we make small, charged molecules from large uncharged fat molecules, the hydrogen atoms are happy to give an electron to the anion (such as acetate) and drift off as a positive hydrogen ion – ie increase acidity.
We always used to rely on measuring urine ketones, but now have a blood ketone meter which is much better at quantifying the amounts in the blood of patients with ketoacidosis. Ryan had a level of 9.6mM when he came in. By the next day he was sitting up, eating lunch, with a level of 0.3mM.

We used to use a sliding scale to treat patients with ketoacidosis. This meant that the higher the blood glucose, the more insulin we gave. It is quite clear from what I have said about what causes ketoacidosis that this was not a sensible strategy. We now give enough insulin (>6units/hr) to completely supress lipolysis until the level of ketones in the blood becomes normal, and usually have to give extra glucose to prevent blood sugar levels going too low.
Now to the food link – butter. As previously discussed, butter is mainly made from triglyceride. It is yellow because it contains vitamin A, or carotene. This helps prevent it becoming oxidised, or rancid.  When butter does goes rancid, it is because the fatty acid chains become oxidised by bacteria, in a process similar to beta-oxidation. One of the main oxidation products of butter is called butyric acid. And, amazingly, the reason butyric acid is called that is because it was first identified in rancid butter – Latin for butter is butyrum (cow-cheese). The simple hydrocarbon with four carbon atoms then became known as butane, again named from butter/butyrum. Not sure why methane, ethane or propane are called that. If I find out I will let you know in a future post.
When butter does goes rancid, it is because the fatty acid chains become oxidised by bacteria, in a process similar to beta-oxidation. One of the main oxidation products of butter is called butyric acid. And, amazingly, the reason butyric acid is called that is because it was first identified in rancid butter – Latin for butter is butyrum (cow-cheese). The simple hydrocarbon with four carbon atoms then became known as butane, again named from butter/butyrum. Not sure why methane, ethane or propane are called that. If I find out I will let you know in a future post.
*cellulose is a glucose polymer made by plants to provide structural support – when it is eaten as food it is known as fibre – it burns well in a calorimeter but cannot be used by humans to provide useful calories – but can be used as an energy source by cows, which, of course, provide us with butter. Ryan likes butter but he does not like fibre. The diabetes dieticians are trying to change that. I wonder if he will be eating brown-bread toast now? (see my very first post – toast and diabetes).