
Kirsty was admitted this morning. She is twenty eight and had been unwell for the last two days. The first thing she noticed was a nasty burning pain when she passed urine. Almost as soon as she had finished peeing she needed to go again. Kirsty went to the doctor yesterday and was prescribed antibiotic tablets. She took one dose and was immediately sick. Then she developed pain in her back, on the left side, under her lower ribs. It started gradually but became almost unbearable. Then she started to feel feverish and shivery. Not ordinary shivery, but uncontrollably shivery – and then she vomited again and again. Her new husband, Sam, drove her up to the emergency department at nine thirty this morning. Their two year old daughter, Ellie, was in the back of the car. In the emergency department Ellie was sitting on Sam’s knee, looking very unconcerned when we talked to Kirsty, who was lying on the trolley.
“I think you have a serious kidney infection” I said, “and we’d better admit you and give you intravenous antibiotics”.
Kirsty was not looking well. She was pale, sweaty, febrile and a bit blotchy with wet hair stuck to her face. She was clutching her painful back with one hand and holding a vomit bowl with the other. She was happy to come in and be looked after. So was Sam. Ellie did not look so sure.

We took a blood sample and blood cultures, gave her intravenous morphine, paracetamol, gentamicin, co-amoxyclav and cyclizine (an antiemetic). She had pyelonephritis – a bacterial infection of her kidney.
Young women get urinary tract infections much more commonly than young men. It’s to do with anatomy. The female urethra is very short, and germs can quite easily travel up to the bladder and then up the ureters to the kidney. Men get urine infections when they are older and have enlarged prostate glands.
Then they cannot empty their bladders completely and the stagnant urine is more likely to become infected. I was taught that urine is normally sterile in healthy people. It seems this is not the case. All of us have bacteria in our urine in small numbers – if you are interested read: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3744036/
We use urine dipstick testing a lot on the acute medical unit. In theory it should be able to tell us which of our patients have a significant urinary tract infection. In practise it is not quite as useful as it should be. Kirsty’s urine tested positive for nitrites and leukocytes. In young women this is a good test, but we knew she had a urinary tract infection anyway. In elderly women, the tests are often positive even if a serious infection is not evident – perhaps because of the innocent commensal bacteria which are present.

How do urine dipsticks work? The test for leukocytes is leukocyte esterase. In infected urine, the leukocytes are polymorphonuclear leukocytes, or neutrophils. I talked earlier (phlegm and horseradish) about how neutrophils, when they get excited by the presence of germs, make the enzyme myeloperoxidase which generates bleach. Esterase is another enzyme neutrophils make which breaks down peptide bonds, and specifically is useful in breaking down the peptidoglycan in bacterial cell walls. I guess its like getting stains out of clothes. Bleach works fine, but the proteolytic enzymes in washing powder can help too.
So what about nitrites? Bacteria like Eschericia coli, which are a common cause of urine infections are known as facultative anaerobes. This means that they can use oxygen to “burn” carbohydrates, protein and fats. But they can also use other “electron acceptors” to do this such as nitrate. Mammalian cells can only use oxygen. When I say mammalian cells, what I mean is mitochondria in mammalian cells – these small structures in our cells are responsible for all the energy generation from glucose, fats and protein. Think what happens when you eat a slice of toast. The amylase in our saliva starts to break down the starch in the toast to form glucose, a process which is finished by amylase in pancreatic secretions. This yields glucose, which is absorbed into the bloodstream. Glucose can be made into energy by the Kreb’s cycle, or citric acid cycle mainly happens in mitochondria. This is a complicated process, but essentially it means that glucose is turned into carbon dioxide and protons and electrons:
The mitochondria don’t get much energy from the Kreb’s cycle, but rely on the protons and electrons produced to make energy by combining them with oxygen. This happens in the electron transport chain:
Bacteria are more versatile than mammalian cells and can get energy out of these protons and electrons, even if oxygen is not available, by using nitrate instead of oxygen:
To do this they need to have the enzyme nitrate reductase. Human cells do not have nitrate reductase, so if nitrate is being turned into nitrite there must be bacteria present. So, if urine contains significant amounts of nitrite, the only way it can get there is if bacteria are using nitrate to “breathe” – a tell-tale sign that infection is present. The reason E.coli is called a facultative anaerobe is that it can survive by making energy if oxygen is present and also when it is not, by using molecules such as nitrate to “breathe”.

When I saw Kirsty later in the afternoon she was much better. The pain had not gone, but was eased by the morphine. Her temperature had come down, and she had stopped vomiting. Her infection was coming under control.
Both gentamicin and co-amoxyclav are very effective in treating urinary tract infections. They are both rapidly excreted by the kidneys and achieve much higher concentrations in the urine than in the bloodstream. Gentamicin has a half-life of about 2 hours in patients with normal renal function. So, lets say we give a person 250mg of gentamicin intravenously. The blood volume is about 5 litres, so the immediate concentration will be 50mg/litre of gentamicin. In two hours about 100ml of urine will be made, and half of the gentamicin previously given intravenously will be in that urine. That is 125mg in 100mls or 1250mg/litre – more than twenty times the concentration in blood. Amoxycillin has a half-life of more like one hour, so achieves even higher urine concentration in comparison to blood.
What do gentamicin and co-amoxyclav do? They are antibiotics that work in quite different ways.
Gentamicin is an aminoglycoside. That means it is a sugar with amine groups. Here is the structure – just three sugars with lots of NH2 groups:
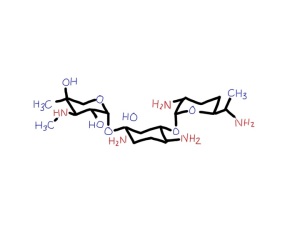
It gets into the bacteria and binds strongly to its ribosomes. These are the really important and clever machines in bacteria which make proteins from DNA. Mammalian cells also have ribosomes to make our proteins – but they are not at all the same. They work in the same way but over the past 2 billion years have changed with evolution so they are a different shape and are larger than bacterial ribosomes. Gentamicin does not interfere with mammalian ribosomes. For an antibiotic to be useful it has to damage bacteria but not human cells. Luckily, ribosomes are so different between bacteria and mammalian cells that some chemicals such as gentamicin will selectively bind only to bacterial ribosomes.

Other antibiotics such as tetracyclines, macrolides (erythromycin and clarithromycin), chloramphenicol and clindamycin also work by interfering with bacterial ribosomal function. A bacterium with damaged ribosomes has major problems – it cannot make new proteins. That means it cannot divide and make new bacteria. It will be immobilised and suffer a slow and painful death. (Not really, I don’t think bacteria don’t feel pain – but then I don’t have evidence for that). If it is a bacterium which makes a protein toxin, such as staphylococcus causing toxic shock syndrome, turning off protein production with a ribosomal poison such as clindamycin is a good idea – rather than causing bacterial cell wall damage and leakage of more toxin with penicillin therapy.
Gentamicin can cause problems if it is given over prolonged periods, because it can accumulate and cause damage to ears and kidneys. The damage to hearing is probably due to damage to mitochondria. More specifically damage to mitochondrial ribosomes. We only gave Kirsty one dose of gentamicin – problems with this drug usually happen when patients with impaired renal function are given aminoglycosides for several days, or when aminoglycosides are given with other drugs such as vancomycin which can impair renal function.
Mitochondria are thought to derive originally from bacteria. Once upon a time, a long time ago there was a cell that survived well enough by getting energy from glycolysis – turning glucose into pyruvate. This cell did not need any oxygen. It scraped a living producing at most 2 ATP molecules per glucose molecule. Then it had a conversation with a bacterium which said “Hi doll, I could take that pyruvate you make and turn it into another 28 ATP molecules by combining it with oxygen – how about it?” Maybe this conversation happened at the time oxygen had begun to appear in the atmosphere (see great oxygenation event in last week’s blog). “With your looks and my talent we could do Broadway together”. This is technically known as endosymbiosis, where one type of cell engulfs another to work together to their mutual benefit. The result is eukaryotic cells – the cells we are made of. Our cells contain mitochondria that derive from bacteria. They can make lots of energy from glucose and oxygen. The bacteria are looked after and nurtured inside the cells which engulfed them. Like bacteria, these mitochondria have their own ribosomes, that, not surprisingly, are similar to the ribosomes of bacteria that are causing Kirsty’s pyelonephritis. Too much gentamicin can damage mitochondrial ribosomes and cause hearing loss – see:
We also gave Kirsty co-amoxyclav.
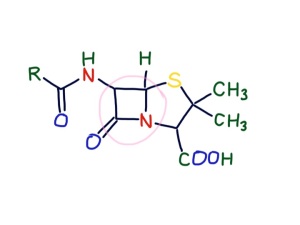
This is a combination of amoxicillin and clavulanic acid. Amoxycillin is a penicillin. Originally discovered by Alexander Fleming, the original penicillin, benzylpenicillin, has been modified by pharmaceutical companies to be more effective. Unlike benzylpenicillin, amoxycillin is rapidly absorbed by the stomach. It is also effective against gram negative organisms such as E.coli. Penicillins have a beta lactam group. This structure makes it difficult for bacteria to make a vital component of their cell wall – peptidoglycan. This is a tough polymer made of special sugars and short peptide chains. The beta-lactam group in penicillins is the right shape to get stuck in the cell wall building enzymes and prevent cell walls being made. Our cells do not have cell walls – they just have thin, delicate plasma membranes made of phospholipid and cholesterol. Similarly, although mitochondria are similar to bacteria, they also do not have cell walls. Mammalian cells and their mitochondria are very cosseted and protected in a 5-star luxury apartment with all mod-cons. They are looked after in a temperature-controlled environment. Oxygen is supplied free and waste carbon dioxide and other unwanted substances taken away continually. Acidity is tightly controlled – pH between 7.35 and 7.45, osmolarity not too high or low. The poor bacterium, in contrast, has to tolerate acid, alkali, high and low osmolarity and a whole host of chemical insults as well as having to find its own food. And then there is the danger of being chased by an angry green neutrophil. No wonder it feels happier with a thick, tough cell wall to protect it. The clavulanic acid is to inactivate beta lactamase – an enzyme some wily bacteria have started making to destroy beta lactam antibiotics. No doubt the bacteria will soon be making betalactamaseinhibitorase enzymes.
There are other ways to selectively attack bacteria without harming human cells. All cells need folate to manufacture nucleic acids. We get our folate from diet – particularly green, leafy vegetables (folate is related to the word foliage). Bacteria do not, in general, have a healthy diet. They instead make the large folate molecule themselves from much smaller molecules. Trimethoprim and sulphonamides prevent bacteria from making folate causing them to suffer and die a “thymineless death” (look it up in Wiki).
Fluoroquinolones such as ciprofloxacin are some of the newer antibiotics which have come into clinical use since I qualified. They were hailed as the new wonder drug, but we now use them relatively rarely because they particularly seem to promote C.difficile infections in frail, elderly people. They work by inhibiting DNA gyrase and Topoisomerase IV. I hope the illustrations will explain how they work:






The food link this week is blue cheese.

The reason it is blue is because of the growth of the mould penicillium, which is a grey/greenish blue colour. The spores are blue, not the fungus itself.

The mould in Roquefort and Stilton is P. roquefortii, a close relative of P. notatum (now known as P. chrysogenum), the penicillium mould that Alexander Fleming found inhibiting the growth of staphylococci.
I’ll be in France next week so the next post will be in 2 weeks’ time